Abstract
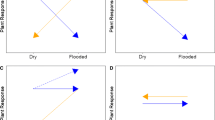
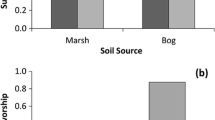
In a tidal marsh on the Savannah River (Georgia, USA), rate of plant community change along a salinity gradient was measured using a reciprocal transplant study. Donor sods were moved in all possible combinations from freshwater to brackish sites and from brackish to freshwater sites at four different locations. The reciprocal aspect of the experiment also allowed the determination of how the rate of plant community change is affected by the direction and level of displacement along the salinity gradient. Stem densities of each species were counted in each transplanted plot in June and October for a 30-month period. Plant community structure and composition changed by a significantly measurable amount within 6 to 18 months of a change in salinity. However, the time required for the transplanted sods to resemble their surrounding communities (at the p≤0.05 level) ranged from 6 to more than 30 months, with some transplanted sods never resembling the surrounding plant communities during the study period. If freshwater or oligohaline sods were moved to more saline environments, environmental conditions appeared to have an overriding effect on the vegetation and community change was rapid, occurring in 6–10 months (mean= 9.3 months, SE=1.9). Shifts from brackish to fresher sites on the salinity gradient delayed community change to about 18 months (mean=15.3 months, SE=1.7) and appeared to be controlled by biotic factors such as vegetative expansion and interspecific competition.
Similar content being viewed by others
Literature cited
Bertness, M. D. and A. M. Ellison. 1987. Determinants of pattern in New England salt marsh plant community. Ecological Monographs 57:129–147.
Brewer, J. S., J. M. Levine, and M. D. Bertness. 1998. Interactive effects of elevation and burial with wrack on plant community structure in some Rhode Island salt marshes. Journal of Ecology 86:125–136.
Brinson, M. M. and R. R. Christian. 1999. Stability of Juncus roemerianus patches in a salt marsh. Wetlands 19:65–70.
Chabreck, R. H. and A. W. Palmisano. 1973. The effects of Hurricane Camille on the marshes of the Mississippi River Delta Plain. Ecology 54:1118–1123.
de la Cruz, A. A. 1981. Differences between South Atlantic and Gulf coast marshes, p. 10–20. In R. Carey, P. S. Markovits, and J. B. Kirkwood (eds.) Proceedings U.S. Fish and Wildlife Service Workshop on Coastal Ecosystems of the Southeastern United States. U.S. Fish and Wildlife Service, Office of Biological Services, Washington, DC, USA. FWS/OBS-80/59.
DeLaune, R. D., S. R. Pezeshki, and W. H. Patrick, Jr. 1987. Response of coastal plants to increase in submergence and salinity. Journal of Coastal Research 3:535–546.
Flynn, K. M., K. L. McKee, and I. A. Mendelssohn. 1995. Recovery of freshwater marsh vegetation after a saltwater intrusion event. Oecologia 103:63–72.
Godfrey, R. K. and J. W. Wooten. 1979. Aquatic and Wetland Plants of Southeastern United States. Monocotyledons. University of Georgia Press, Athens, GA, USA.
Godfrey, R. K. and J. W. Wooten. 1981. Aquatic and Wetland Plants of Southeastern United States. Dicotyledons. University of Georgia Press, Athens, GA, USA.
Guntenspergen, G. R., D. R. Cahoon, J. Grace, G. D. Steyer, S. Fournet, M. A. Townson, and A. L. Foote. 1995. Disturbance and recovery of the Louisiana coastal marsh landscape from the impacts of Hurricane Andrew. Journal of Coastal Research SI21:324–339.
Hacker, S. D. and M. D. Bertness. 1999. Experimental evidence for factors maintaining plant species diversity in a New England salt marsh. Ecology 80:2064–2073.
Hartman, J. M. 1988. Recolonization of small disturbance patches in a New England salt marsh. American Journal of Botany 75:1625–1631.
Howard, R. J. and I. A. Mendelssohn. 1999a. Salinity as a constraint on growth of oligohaline marsh macrophytes. I. Species variation in stress tolerance. American Journal of Botany 86:785–794.
Howard, R. J. and I. A. Mendelssohn. 1999b. Salinity as a constraint on growth of oligohaline marsh macrophytes. II. Salt pulses and recovery potential. American Journal of Botany 86:795–806.
Latham, P. J., L. J. Pearlstine, and W. M. Kitchens. 1991. Spatial distributions of the softstem bulrush, Scripus validus across a salinity gradient. Estuaries 14:192–198.
McCune, B. and J. B. Grace. 2002. Analysis of Ecological Communities. MjM Software Design, Gleneden Beach, OR, USA.
McCune, B. and M. J. Mefford. 1999. PC-ORD. Multivariate Analysis of Ecological Data, Version 4. MjM Software Design, Gleneden Beach, OR, USA.
McKee, K. L. and I. A. Mendelssohn. 1989. Response of a freshwater marsh plant community to increased salinity and increased water level. Aquatic Botany 34:301–316.
Mielke, P. W., Jr. and K. J. Berry. 2001. Permutation Methods: a Distance Function Approach. Springer, New York, NY, USA.
Mitsch, W. J. and J. G. Gosselink. 2000. Wetlands, 3rd ed. Van Nostrand Reinhold, New York, NY, USA.
Odum, W. E., T. J. Smith II, J. K. Hoover, and C. C. McIvor. 1984. The ecology of tidal freshwater marshes of the United States east coast: a community profile. U.S. Fish and Wildlife Service, Washington, DC, USA. FWS/OBS-83/17.
Odum, W. E. 1988. Comparative ecology of tidal freshwater and salt marshes. Annual Review of Ecology and Systematics 19:147–176.
Pearlstine, L. G., W. M. Kitchens, P. J. Latham, and R. D. Bartleson. 1993. Tide gate influences on a tidal marsh. Water Resources Bulletin 29:1009–1019.
Pennings, S. C. and R. M. Callaway. 1992. Salt marsh plant zonation: the relative importance of competition and physical factors. Ecology 73:681–690.
Roman, C. T., W. A. Niering, and R. S. Warren. 1984. Salt marsh vegetation change in response to tidal restriction. Environmental Management 8:141–150.
Snow, A. A. and S. W. Vince. 1984. Plant zonation in an Alaskan salt marsh. II. An experimental study of the role of edaphic conditions. Journal of Ecology 72:669–684.
Visser, J. M., C. E. Sasser, R. H. Chabreck, and R. G. Linscombe. 1999. Long-term vegetation change in Louisiana tidal marshes, 1968–1992. Wetlands 19:168–175.
Warren, R. S. and W. A. Niering. 1993. Vegetation change on a northeast tidal marsh: interaction of sea-level rise and marsh accretion. Ecology 74:96–103.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wetzel, P.R., Kitchens, W.M., Brush, J.M. et al. Use of a reciprocal transplant study to measure the rate of plant community change in a tidal marsh along a salinity gradient. Wetlands 24, 879–890 (2004). https://doi.org/10.1672/0277-5212(2004)024[0879:UOARTS]2.0.CO;2
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1672/0277-5212(2004)024[0879:UOARTS]2.0.CO;2